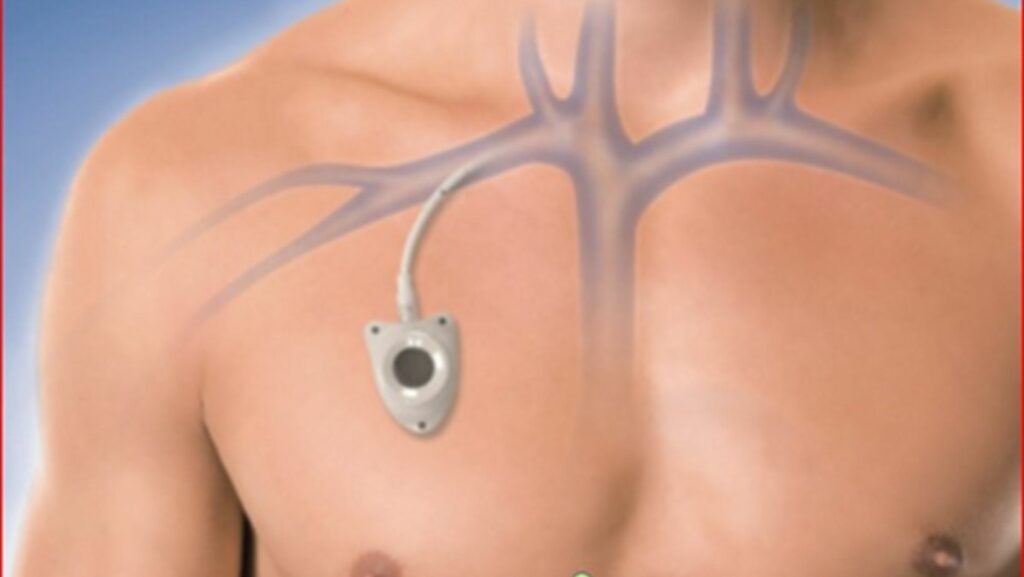
The Bard PowerPort is a high-quality implantable port device designed for patients requiring frequent and long-term intravenous access. Marketed for its durability and ease of use, the PowerPort allows healthcare providers to administer medications, draw blood, and perform IV therapies with minimal discomfort to the patient.
However, this medical innovation has come under scrutiny due to numerous reports of complications, leading to a significant lawsuit against Bard. This legal action sheds light on various issues users have faced, casting shadows on its advertised functionalities. Through this article, we aim to explore the flaws identified in the lawsuit, offering a comprehensive understanding of the challenges patients and healthcare providers have encountered.
For those interested in the details of the legal challenges and testimonies, speak with a lawyer to learn more about the Bard PowerPort lawsuit and grasp the full scope of the controversies surrounding this medical device. Our goal is to present an informed analysis of the situation, ensuring our readers are well-educated on the matter at hand.
So, let’s explore the specifics of the flaws found in the Bard PowerPort lawsuit.
Background on Bard PowerPort
Before we discuss the specifics of the lawsuit, let’s first understand what the Bard PowerPort is and who it is intended for.
According to the manufacturer, Bard PowerPort is a fully implantable device that provides easy access to the bloodstream for delivering medications and fluids. This eliminates the need for repeated needle insertions, making treatment more comfortable for patients.
Initially marketed towards individuals who require frequent medication or intravenous (IV) therapy, the Bard PowerPort gained traction for its ease of use and potential to improve patients’ quality of life. Positive reviews and strong initial sales figures seemed to indicate a successful product launch.
However, the situation took a turn when reports of complications and adverse events started surfacing, leading to the filing of the lawsuit against Bard.
The Lawsuit Against Bard
The lawsuit against Bard involves plaintiffs ranging from individual users who experienced direct complications from the Bard PowerPort to consumer protection agencies advocating for affected individuals. These parties have come together to file a suit against Bard, grounding their claims in the alleged misrepresentation of the device’s functionalities, performance issues not aligning with advertised promises, and serious safety hazards associated with PowerPort’s use.
Central to the lawsuit is the allegation that Bard misrepresented the safety and efficiency of the PowerPort, claiming it to be a reliable and hassle-free solution for patients requiring long-term intravenous treatments. Plaintiffs argue that instead of offering a safer, more comfortable experience, the PowerPort presented significant health risks, including device failure, infections, and complications leading to additional medical interventions.
 The legal grounds for the lawsuit encompass claims such as breach of warranty, failing to ensure the device performed as warranted, and false advertising, marketing the product in a deceptive manner that obscured the potential risks and overemphasized its capabilities. This litigation aims to seek reparations for the affected parties, hold Bard accountable for its practices, and ensure higher safety standards for medical devices.
The legal grounds for the lawsuit encompass claims such as breach of warranty, failing to ensure the device performed as warranted, and false advertising, marketing the product in a deceptive manner that obscured the potential risks and overemphasized its capabilities. This litigation aims to seek reparations for the affected parties, hold Bard accountable for its practices, and ensure higher safety standards for medical devices.
Examining the Alleged Flaws
Through this lawsuit, we get a closer look at Bard PowerPort’s alleged flaws. Let’s examine some of the main issues raised by the plaintiffs and how they have impacted patients and healthcare providers.
Device Failure
One of the most significant concerns highlighted in the lawsuit is device failure. Reports indicate numerous users experienced malfunctioning PowerPorts, leading to additional surgeries and procedures. This can have serious consequences for patients who require uninterrupted access to their bloodstream, potentially causing delays in treatment or even jeopardizing their health.
Infections
Another critical flaw identified in the lawsuit is the risk of infections associated with using Bard PowerPort. Plaintiffs argue that Bard failed to adequately warn users about the potential infection risks, which can be life-threatening for individuals with compromised immune systems. This has been a major concern for healthcare providers, who have to closely monitor patients and take additional precautions when using the PowerPort.
Migration or dislodgement
The lawsuit also highlights concerns regarding the PowerPort’s migration or dislodgement from its intended placement site. This not only causes discomfort and pain for patients but can also lead to additional surgical procedures and potential complications.
Potential Impact of the Lawsuit
The lawsuit against Bard has already had a significant impact, both on the company itself and the medical community as a whole. The legal action has brought attention to the flaws in the PowerPort and raised awareness about potential risks associated with its use.
This heightened focus on patient safety and device performance has resulted in increased scrutiny from regulatory bodies, potentially leading to stricter regulations for medical devices such as the PowerPort.
This could ultimately benefit patients by ensuring higher safety standards and more reliable products.
Are You Affected by the Bard PowerPort Lawsuit?
If you or a loved one has experienced complications or adverse effects from using the Bard PowerPort, you may be eligible to join the lawsuit and seek reparations for your losses. It’s essential to consult a lawyer specializing in medical device litigation to understand your legal options and determine if you have a case against Bard.
Your health and well-being should always be a top priority, and taking legal action can help you seek justice and hold companies accountable for their actions. Stay informed about the developments in this lawsuit as it progresses, and remember to prioritize your safety when making medical decisions.












